For more than 20 years, the Stem Cell Network has made strategic investments in stem cell and regenerative medicine research, helping to position Canada as a global leader in the field. As Canada’s only national stem cell and regenerative medicine network, SCN’s research funding translates Canadian regenerative medicine research discoveries from the innovation and developmental stages through pre-clinical evaluation and into clinical trials and commercial applications.
SCN funds translational research through a suite of funding programs. Through these award programs, SCN powers stem cell and regenerative medicine research across the clinical pipeline in more than 26 disease areas, including heart disease, diabetes, cancer, and neurological diseases.
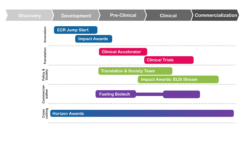
Over the course of the 2022–2025 period, two peer-reviewed funding competitions were held to identify the research projects to be funded. The first competition kicked off in September 2021 and offered two- and three-year awards that began in April 2022. The second competition launched September 1, 2022 and offered 22 month awards that began in April 2023. Between 2022 and 2025, SCN is supporting 56 research projects — including clinical trials.
SCN’s Peer Review Process
To ensure the research projects and clinical trials SCN funds align with our strategic mandate, SCN employs a two-stage peer review process that first evaluates the scientific excellence of an application and then assesses the strategic fit with our mandate and contractual obligations to the Government of Canada:
Stage one: Applications are evaluated to identify scientifically excellent research that will further the field, is novel and will ensure Canada continues to have international impact within the area of regenerative medicine. The evaluation of the scientific merit of an application is performed by SCN’s international peer review committees, comprising international subject matter experts drawn from academia and industry. For the 2023 funding competition, three international peer review committees were utilised: the Translational Research Committee, the Policy Review Committee and the International Scientific Advisory Board. The peer reviewers who served on these committees in 2023 are listed here.
Stage two: A strategic evaluation of highly ranked peer reviewed applications is conducted by SCN’s Research Management Committee (RMC). The RMC is responsible for considering how each potential project aligns with SCN’s mandate, budget needs, partner support, project potential for clinical and/or commercial translation, HQP training, knowledge mobilization, and the fostering of equity, diversity and inclusion within the multi-disciplinary research team.
Based on the Stage one scientific peer review and the Stage two strategic evaluation, the RMC and SCN Management make project funding recommendations for approval by SCN’s Board of Directors.
SCN uses a Continual Review process for all projects approved by SCN’s Board of Directors to ensure a research project’s milestones and deliverables are being met and that SCN funds are appropriately invested. Continual Review is conducted by the RMC, which offers insight and direction in reviewing the annual project progress reports. The RMC may also directly meet with investigators to better understand the nuances of their research so investigators receive the best possible feedback and support.