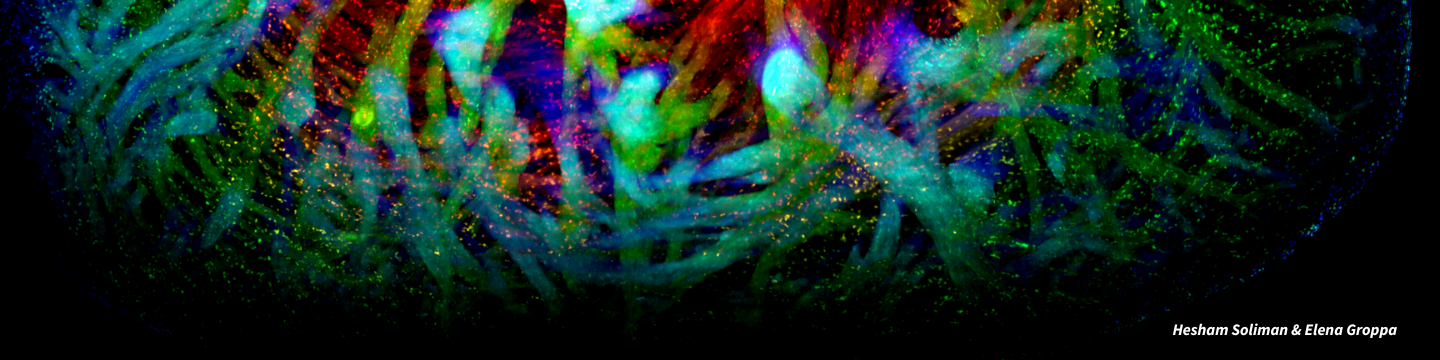
Stem cells are our bodies’ building blocks. They are different from other cells because they have the potential to differentiate into any cell type and can renew themselves.
Because stem cells can repair tissue and organs, they are powering regenerative medicine (RM), a new way to treat disease and injury. RM is considered by investors, economists and health policy experts to be the next frontier of modern medicine.
The power of RM is in its potential to halt or reverse disease instead of simply alleviating symptoms, which is unlocking leading-edge treatments for diseases such as, type 1 diabetes, cancer, muscular dystrophies, lung and heart disease, as well as for neurological diseases such as Parkinson’s and Alzheimer’s.

Drs. James Till and Ernest McCulloch
Canada has been a world leader in RM from the start. In the 1960s, Canadian scientific pioneers Drs. James Till and Ernest McCulloch definitively confirmed the existence of stem cells while investigating the effects of radiation on mouse bone marrow. Their seminal paper unlocked a field of research with potential to tackle some of our greatest health challenges and established Canada as a global leader in stem cell and regenerative medicine research. Since then, Canadian researchers have continued to make significant contributions in this field.

Read more about the potential of stem cells and regenerative medicine in SCN’s recent publication, Regenerative Medicine in Canada